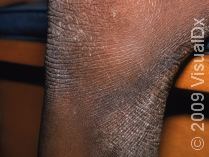
Finally, there is a new hope for adult patients suffering from moderate to severe atopic dermatitis (AD), or eczema. Both dermatologists and AD patients can celebrate. Atopic dermatitis affects up to 20% of children in the United States. Although the vast majority outgrow their disease, approximately 10% of individuals never do. They continue to have atopic dermatitis into adulthood. Often times, this can be debilitating. The itch can be uncontrollable. Sufferers can’t get a good night of sleep.
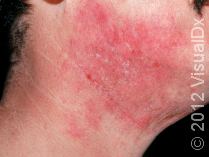
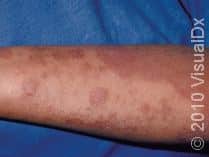
Before the approval of dupilumab (Dupixent) by the Food and Drug Administration (FDA) on March 28th, 2017, there were limited treatments. Topical creams and ointments could only do so much. If topical treatments weren’t enough, patients had to rely on phototherapy. Although phototherapy is affective for AD, not everyone can make it to a doctor’s office three times a week for therapy. The other oral medications can be toxic after long-term use or have serious side effects.
Dupilumab is an injectable monoclonal antibody that blocks the receptor of a special signaling molecule (interleukin [IL] 4). By blocking the IL-4 receptor, dupilumab also blocks signaling from another molecule (IL-13). Scientists believe both IL-4 and IL-13 are two key drivers of AD.
How Did the FDA Decide to Approve Dupilumab?
A total of more than 1,379 patients were studied in two major clinical trials. Dupilumab was tested against placebo. The main endpoint of the clinical studies was how many doctors rated patient improvement as “clear” or “almost clear.” About 36-38% of patients receiving dupilumab achieved a rating of “clear” or “almost clear” compared with only 8% of patients receiving a placebo. About half of patients with AD improved at least 75% on the medication compared with only 12-15% on the placebo. The medication improved itching, depression, and quality of life for patients. The clinical trials were pretty convincing. The medication works, and it works well.1
What Risks Are There for Dupilumab?
With all new medications, we have less data. This is something to keep in mind. Sometimes, serious side effects or adverse events are only apparent years after a drug enters the market. There will be a lot of unknowns as doctors have more experience with the medical problems.
From the clinical trials, dupilumab did increase eye problems. Specifically, patients on dupilumab should be aware that there is an increased risk for pink eye, or conjunctivitis, and keratitis, or corneal inflammation. The FDA warns patients on the medication that if they do experience eye itching, redness, visual changes, or pain, they should tell their doctor right away.
What’s Next for Dupilumab?
The long-term safety and effectiveness of dupilumab has not been established yet. More data is needed on how long the benefit lasts with dupilumab and whether there are safety concerns that have not yet become apparent based on the clinical trials.
The medication is also only approved for adults. More clinical trials must be conducted before the medication can be approved for pediatric patients. Beyond AD, dupilumab shows promise for other allergic diseases such as asthma. However, the FDA has not yet approved the medication for other allergic diseases.
Bottom Line
Dermatologists and patients finally have a new weapon against moderate to severe AD. Twenty years ago, psoriasis seemed to be another skin disease that patients had to “live with.” When new biologics became readily available for psoriasis in the late 2000s, dermatologists were able to provide much better disease control. Taming psoriasis represents one of the most important and impressive medical achievements in the 21st century. We can only hope that with dupilumab and other new drugs still in development, we will be able to tame eczema too.
References
1. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335-2348. [PubMed]
Last modified on October 18th, 2018 at 6:46 pm